Specification
- सटीकता
- High (membrane separation)
- शेल्फ लाइफ
- 3-5 years (unopened, sterile)
- उपयोग का प्रकार
- Hospital/Clinical
- माप सीमा
- Ultrafiltration rate up to 0.2 L/min
- फंक्शन
- Removes excess plasma water and waste products from blood
- इंस्ट्रूमेंट्स टाइप
- Extracorporeal blood purification device
- विशेषताएँ
- High ultrafiltration rate, high clearance for plasma water, low priming volume, biocompatible and pyrogen-free
- स्टोरेज निर्देश
- Store in cool, dry place away from sunlight
- मटेरियल
- शर्त
- टेक्नोलॉजी
- Hollow fiber membrane filtration
- पोर्टेबल
- Yes
- वॉल माउंटेड
- रियल-टाइम ऑपरेशन
- शोर का स्तर
- Silent operation
- ऑपरेटिंग टाइप
- उपयोग करें
- Hemoconcentration during Cardiopulmonary Bypass
- पावर सोर्स
- आयाम (एल* डब्ल्यू* एच)
- Approx. 300 mm length, 42 mm diameter
- वज़न
- Approx. 220 g
- रंग
- Transparent/White
- Maximum Transmembrane Pressure
- 500 mmHg
- Compliance Standard
- CE Marked, ISO certified
- Single Use
- Yes (disposable)
- Priming Volume
- 45 mL
- Connector Type
- Standard Luer lock / tubing connections
- Blood Flow Rate
- 100 to 700 mL/min
- Intended Patient Population
- Adults/Children (as per clinical guidance)
- Endotoxin Retention
- High
- Latex Content
- Latex-free
- Sterilization Method
- ETO Sterilized
- Membrane Surface Area
- 0.2 m²
- Operating Temperature
- 15°C – 37°C
About
ADULT : EFFECTIVE SURFACE AREA 0.9 m2 AND FOR THAT MINIMUM PRIMING VOLUME 44 ml , MTP 600 mmHg
PEDIATRIC : EFFECTIVE SURFACE AREA 0.6 m2 AND FOR THATMINIMUM PRIMING VOLUME 32 ml , MTP 600 mmHg
NEONATAL: EFFECTIVE SURFACE AREA 0.2 m2 AND FOR THATMINIMUM PRIMING VOLUME 18 ml , MTP 600 mmHg
USER
For reference please note some hospitals as like UN Mehta Institute of Cardiology and ResearchCentre (Ahmedabad), Geetanjali Medical College & Hospital (Udaipur),NARAYAN HOSPITAL ( MUMBAI ) and many more counting ...
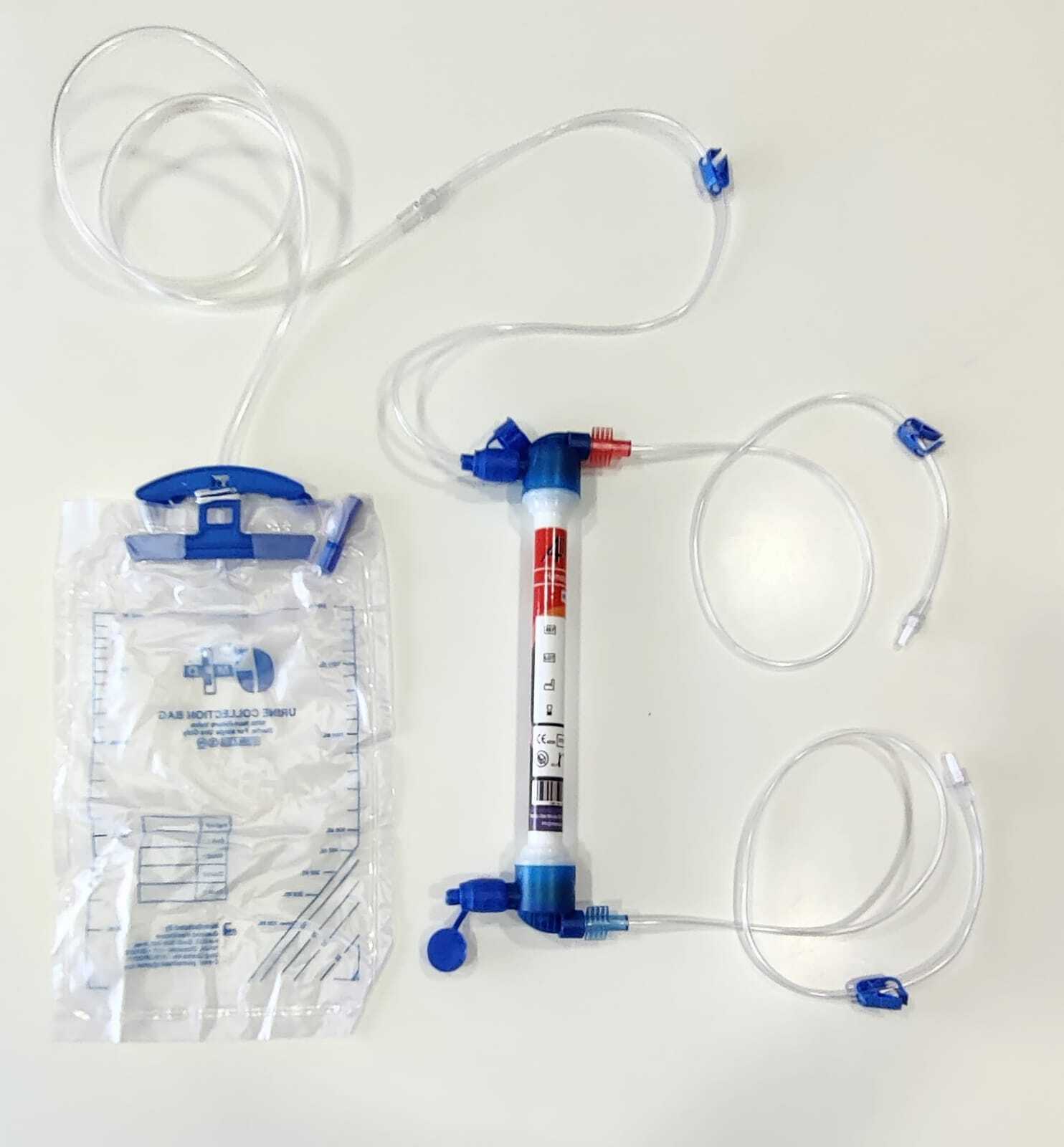
Advanced Hemoconcentration Technology
Utilizing hollow fiber membrane filtration, the Hemoconcentrator 0.2 delivers superior ultrafiltration and plasma water clearance. Its high-end PES membrane ensures efficient separation of plasma while retaining endotoxins, offering reliable, pyrogen-free purification during extracorporeal procedures. The device is suitable for adults and children, ensuring versatile clinical application.
Safety and Compliance
Manufactured from polycarbonate/polypropylene materials and sterilized with ethylene oxide (ETO), this device is latex-free, minimizing allergic reactions. It adheres to stringent CE and ISO standards, guaranteeing high accuracy and biocompatibility for patient safety. Its long shelf life and stable operation make it ideal for hospital and clinical use.
User-Friendly and Portable Design
The Hemoconcentrator's transparent, lightweight construction allows for effortless portability and integration in busy clinical environments. With standard Luer lock connectors, silent operation, and no external power requirements, clinicians can operate it manually or with an extracorporeal circuit for real-time blood water removal, enhancing workflow efficiency.
FAQ's of Hemoconcentrators 0.2:
Q: How is the Hemoconcentrator 0.2 used during cardiopulmonary bypass procedures?
A: The Hemoconcentrator 0.2 is connected to the extracorporeal blood circuit via standard Luer lock or tubing connections. It operates using the circuit's pressure to separate and remove excess plasma water and waste products, aiding in the management of fluid balance during cardiopulmonary bypass.Q: What types of patients can benefit from this hemoconcentrator?
A: This device is designed for both adult and pediatric patients, as guided by clinical protocols. Its adjustable blood flow rate and high biocompatibility make it suitable for a wide range of clinical scenarios requiring extracorporeal blood purification.Q: When should the hemoconcentrator be replaced or disposed of?
A: The Hemoconcentrator 0.2 is intended for single use only. It must be disposed of after each procedure to prevent contamination, in accordance with hospital waste management practices.Q: Where should the Hemoconcentrator 0.2 be stored before use?
A: Store the device in a cool, dry place away from sunlight. Unopened and sterile units have a shelf life of three to five years under recommended storage conditions.Q: What is the process for setting up the Hemoconcentrator 0.2 in a clinical environment?
A: Setup involves connecting the device's Luer lock or tubing connectors to the blood circuit, priming with approximately 45 mL, and ensuring proper temperature and operating conditions (15C-37C) before initiating ultrafiltration.Q: What are the main benefits of using this hemoconcentrator?
A: Benefits include highly effective plasma water removal, reliable endotoxin retention, low priming volume, silent operation, high accuracy, and compatibility with standard hospital protocols-improving patient outcomes during extracorporeal procedures.Q: Does the Hemoconcentrator 0.2 require an external power source for operation?
A: No external power source is needed; the device uses the blood circuit's pressure for real-time ultrafiltration, supporting seamless integration with manual or assisted extracorporeal systems.
Tell us about your requirement

Price: Â
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
मोबाइल number
Email



 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese